Answer:
Approximately
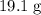 .
.
Step-by-step explanation:
Number of moles of formula units of magnesium sulfate required to make the solution
The unit of concentration in this question is "
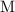 ". That's equivalent to "
". That's equivalent to "
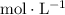 " (moles per liter.) In other words:
" (moles per liter.) In other words:
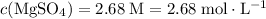 .
.
However, the unit of the volume of this solution is in milliliters. Convert that unit to liters:
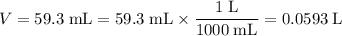 .
.
Calculate the number of moles of
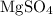 formula units required to make this solution:
formula units required to make this solution:
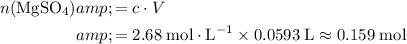 .
.
Mass of magnesium sulfate in the solution
Look up the relative atomic mass data of
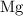 ,
,
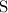 , and
, and
 on a modern periodic table:
on a modern periodic table:
Calculate the formula mass of
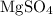 using these values:
using these values:
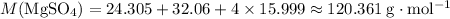 .
.
Using this formula mass, calculate the mass of that (approximately)
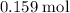 of
of
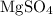 formula units:
formula units:
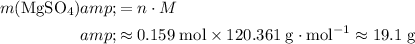 .
.
Therefore, the mass of
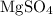 required to make this solution would be approximately
required to make this solution would be approximately
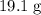 .
.