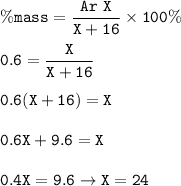Atomic weight of metal : 24
Further explanation
Proust states the Comparative Law that compounds are formed from elements with the same Mass Comparison so that compounds have a fixed composition of elements
Divalent metal oxide=XO
MW O = 16
MW XO₂ = X+16