Answer:
V2=3.482L
Step-by-step explanation:
The reaction takes place as follows
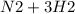 →
→
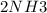
In order to find the volume of this sample after the reaction we will use the
Avogadro's law
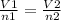
V1=17.4L
n1= moles of N2 +3H2= 0.1+3*0.3=1mole
V2= to be found
Now to calculate the n2 of NH3 we will use the reaction
as we know that N2 is limiting reagent and hydrogen is present in excess so the ammonia will only be formed by this limiting nitrogen
1mole of N2 gives 2 mole of NH3
0.1*2= 0.2mole of NH3 will be produced
n2 = 0.2
upon substituting theses values in the Avogadro law
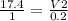
V2=3.482L