Answer:
Computed value is 45.3 kJ/mol whereas the accepted one is 32.6 kJ/mol which means there is a significant difference between them.
Step-by-step explanation:
Hello.
In this case, for this problem we write the following equation, representing that the heat released due to the combustion of cyclopentane equals the heat gained by the calorimeter:
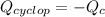
Which can be also written as:
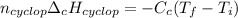
In such a way, we can compute the enthalpy of combustion of cyclopentane:
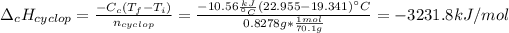
Next, since the combustion of cyclopentane is:
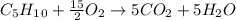
And the enthalpy of combustion is computed thermochemically as:
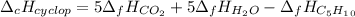
Since the enthalpy of formation of CO2 and H2O are -395.5 and -241.8 kJ/mol respectively, we can compute the enthalpy of formation of cyclopentane based on the previously computed enthalpy of combustion on the calorimeter part:
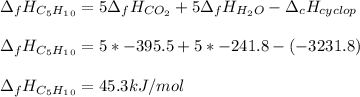
In such a way, we see a significant difference between the computed value 45.3 kJ/mol and the accepted value 32.6 kJ/mol.
Best regards.