The complete question is
For a hydrogen-like atom, classify these electron transitions by whether they result in the absorption or emission of light.
n=3 to n=2
n=3 to n=5
n=1 to n=3
n=2 to n=1
Ignoring sign, which transition is associated with the greatest energy change
Answer:
Absorption n=1 to n = 5, n = 3 to n = 5
Emission n= 3 to n= 2, n = 2 to n= 1
When you will calculate the energy difference from these transition using the formula
E =
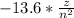
Delta E(n=3 to n= 2) = 13.6* 5/36 = 1.888eV
Delta E (n=3 to n= 5) = 0.967eV
Delta E ( n= 1 to n= 3) = 12.088eV
Delta E ( n= 2 to n=1) = 10.2eV
clearly n=1 to n = 3 has the greatest energy.