Answer:
2.676e22 atoms of Hydrogen
Step-by-step explanation:
Knowing the density of water is 1g/ml, 1(g/ml)*0.4ml=0.4 grams of H2O. Knowing that there are 16 grams of H2O in 1 mole of H2O, we can set up a unit conversion of 0.4 Grams H2O *
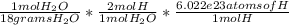 =
=
2.676e22 atoms of Hydrogen.