Answer:
0.2moles
Step-by-step explanation:
Given parameters:
Number of molecules of H₂ = 1.8 x 10²³molecules
Unknown:
Number of moles of NH₃ = ?
Solution:
The reaction equation is given as:
N₂ + 3H₂ → 2NH₃
Let us solve from the known to the unknown specie;
Since the number of molecules is 1.8 x 10²³molecules of H₂;
6.02 x 10²³molecules will give 1 mole of a substance
1.8 x 10²³molecules will produce
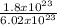 mole of H₂
mole of H₂
= 0.3moles of H₂
From the balanced chemical equation;
3 moles of H₂ produced 2 moles of NH₃
0.3 moles of H₂ will yield
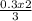 = 0.2moles
= 0.2moles