Answer:
0.0132 g CuSO₄
General Formulas and Concepts:
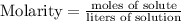
Step-by-step explanation:
Step 1: Define variables
3.29 mL
4.00 M CuSO₄
x g CuSO₄
Step 2: Define conversions
1000 mL = 1 L
Step 3: Solve
- Convert mL to L:
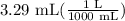 = 0.00329 L
= 0.00329 L - Substitute:
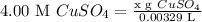
- Multiply both sides by L:
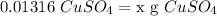
- Rewrite:
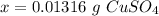
Step 4: Simplify
We are given 3 sig figs.
0.01316 g CuSO₄ ≈ 0.0132 g CuSO₄