Answer:
48dm³
Step-by-step explanation:
Given reaction:
Ca(OH)₂ + 2HBr → CaBr₂ + 2H₂O
Parameters:
Concentration of HBr = 0.5M
Volume of Ca(OH)₂ = 40mL
Concentration of Ca(OH)₂ = 0.3M
Solution:
To solve this problem, we are going to use the mole concept. We solve from the known specie to the unknown.
We first find the number of moles of the known specie which is the Ca(OH)₂ ;
number of moles = concentration x volume
number of moles = 0.3 x 40 x 10⁻³ = 0.012moles
From the reaction equation;
1 mole of Ca(OH)₂ requires 2 moles HBr
0.012 moles of Ca(OH)₂ will require 0.012 x 2 = 0.024moles of HBr
Now,
To find the volume of HBr;
Volume =
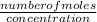
Volume =
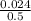 = 0.048dm³
= 0.048dm³
In mL;
Volume 0.048 x 1000 = 48dm³