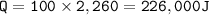The quantity of heat : 226,000 J
Further explanation
Heat can be calculated using the formula:
Q = mc∆T
Q = heat, J
m = mass, g
c = specific heat, joules / g ° C
∆T = temperature difference, ° C / K
Also can be formulated :
Q = m. Hf
Hf = heat of fusion
Heat required for vaporize /evaporated can be calculated from
Q = m . Hv
m = mass, g
Hv = the heat of vaporization, for water = 2,260 J/g
So :