Answer:
Only A., the stick structure with no branches and five vertices between the two endpoints.
Step-by-step explanation:
An alkane is a saturated hydrocarbon compound with no branches- just a series of carbon atoms connected with
 single bonds. Heptane is the name for an alkane with
single bonds. Heptane is the name for an alkane with
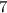 carbon atoms (the prefix "
carbon atoms (the prefix "
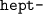 " means "seven".)
" means "seven".)
Notice that there are only
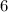 carbon atoms in the alkane with the condensed structure formula
carbon atoms in the alkane with the condensed structure formula
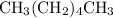 . The name for that structure would be hexane, not heptane.
. The name for that structure would be hexane, not heptane.
In general, the condensed structure formula of an alkane with
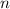 carbon atoms (
carbon atoms (
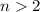 ) is
) is
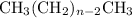 . The correct condensed structure formula for heptane (
. The correct condensed structure formula for heptane (
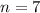 ) would be
) would be
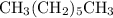 .
.
The structure in choice A is represented as a line-angle formula. As long as no letter is present in the formula, each vertex in the line-angle formula (including the endpoints) represents a carbon atom. Hence, the line-angle formula in choice A would represent a hydrocarbon with
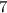 carbon atoms.
carbon atoms.
A single line denotes a single bond between atoms. All
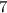 carbon atoms in this structure are thus joined in a line with
carbon atoms in this structure are thus joined in a line with
 single bonds. Hence, this structure denotes an alkane with
single bonds. Hence, this structure denotes an alkane with
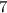 carbon atoms- heptane.
carbon atoms- heptane.