Answer:
Limiting Reagent or Limiting Reactant
Step-by-step explanation:
- The limiting reagent of a reaction is the reactant that runs out first. Once it is completely consumed, the reaction stops.
- The limiting reagent is the only chemical that is used to calculate the theoretical yield. It is used up first. After that, any excess reagent will not be able to produce more products.
And to assess which reactant is which, we must usually have quantities of each reagent in a chemical reaction...and of course we should write a stoichiometric equation that is balanced with respect to mass and charge in order to assess the stoichiometric equivalence.
We could examine for instance, the combustion of methane gas, the which MAY have cooked your dinner … its combustion reaction is as shown...
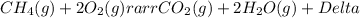
And here conditions of COMPLETE combustion pertain; there is DIOXYGEN in excess, AND methane is the limiting reagent. A
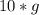 mass of methane represents a molar quantity of …
mass of methane represents a molar quantity of …
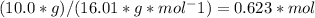
And with respect to dioxygen we thus require...

...but this quantity (AND MORE) is available from the atmosphere....and the methane was the limiting reagent....