Step-by-step explanation:
INTRODUCTION:
There are 3 main subatomic particles, they are:
- Electrons
- Protons
- Neutrons
The existence of the sub-atomic particles was proved by the fact that an atom is electrically neutral but it can be made to gain a positive or a negative charge. This means that an atom must contain tiny particles, each carrying either a positive or a negative charge.
These opposing charges balance each other under ordinary conditions to make an atom electrically neutral.
ELECTRONS:
Electrons were discovered in 1897 by J.J. THOMSON when he was studying cathode rays.An electron is denoted by:
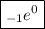
Where,
- 0 = represents it's mass
- -1 = represents it's unit negative electrically charge.
Properties of electrons:
- An electron is a negatively charged particle
- They are an integral part of all atoms
- The properties of electrons are Independent of the nature of the gas in the discharge tube
- An electron has a mass of 9-108 x 10 g which is only 1/1837 of the mass of a hydrogen atom. Therefore, an electron is said to have negligible mass
- An electron carries one unit negative (-1) charge, which is equal to 1-602 x 10 coulombs.
UNIT CHARGE OF Electrons:
NEUTRONS:
In 1932, James chadwick discovered neutrons & it had no charge. Its denoted as
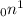
PROPERTIES OF NEUTRONS:
- Electrically a neutron is neutral, i.e it has no charge
- Neutrons are present in the nucleus of an atom
- Atoms of the same element may differ in the number of neutrons leading to formation of isotopes.
An atom of hydrogen contains only 1 proton and one electron but no neutron.
UNIT CHARGE OF IT:
PROTONS:
An atom is electrically neutral Therefor the presence of the negatively charger electrons in an atom suggests that it must contain positively charged particles a well, otherwise an atom would not b electrically neutral.It is discovered by E. GOLDSTEIN.
It's denoted as
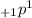
where 1 represents 1 a.m.u. and +1 represents 1 unit positive charge.
PROPERTIES:
- A proton is a positively charged particle.
- The mass of a proton is 1.672 × 10^(-19). ie equal to the mass of an atom of hydrogen
- A proton carries 1 unit positive (+1) charge , and equals to 1.602 × 10^(-19) coulombs.
>>>All elements contains electrons & protons.
Since an atom is electrically neutral, the number of electrons in an atom is equal to the mumber of protons in that atom. However, no two elements contain the same number of protons in their respective nuclei.
UNIT Charge:
[All units I provided are a.m.u. .]

Hope this helps :)