Answer:
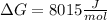
Step-by-step explanation:
Hello.
In this case, for the following chemical reaction in gaseous phase:
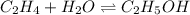
We can write the equilibrium expression as:
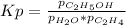
it means that given the pressures, we can compute Kp as shown below:
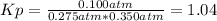
Next, we compute Kc at 298 K:
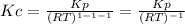
Whereas 1-1-1 comes from the stoichiometric coefficients of products minus reactants. Therefore, Kc is:
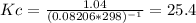
Thus, the Gibbs free energy for this reaction is:
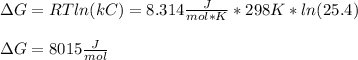
Best regards!