Answer:
Diffusion coefficient D = 2.6613 × 10⁻¹¹ m²/s
Step-by-step explanation:
From the given information:
Carbon concentration
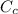 = 0.012
= 0.012
Iron concentration
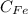 = 100 - 0.012 = 99.988
= 100 - 0.012 = 99.988
Density of carbon = 2.25 g/cm³
Density of iron = 7.87 g/cm³
We can convert the carbon concentrations from weight percentage to kilograms carbon per meter cubed by using the formula.
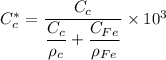
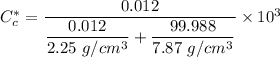
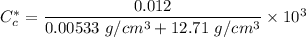
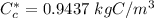
However; for 0.0069 wt% C
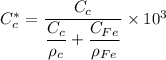
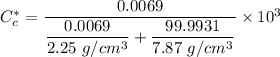
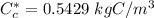
Thus; the diffusion coefficient can be computed by using the formula:
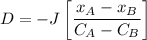
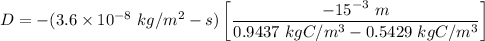
D = 2.6613 × 10⁻¹¹ m²/s