Answer: Root mean square speed, Vrms=5,694m/s
Step-by-step explanation:
Root mean square speed is defined as the the square root of the average of the square of the velocity and given as
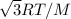
Where R =8.3145J/mol.k
M molar mass of the molecule in kg/mol
Helium= He=4g/mol = 0.004kg/mol
T= Temperature = 5200k
Vrms=
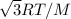
=
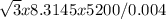
=
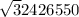
Vrms =5,694m/s