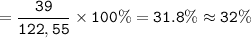The percent of potassium in the compound KClO₃ = 32%
Further explanation
Proust states the Comparative Law that compounds are formed from elements with the same Mass Comparison, so that compounds have a fixed composition of elements
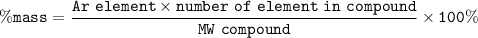
We determine the molecular mass of KClO₃ then we determine the Ar from K (potassium) and the amount in the compound
MW KClO₃ : 122,55 g/mol
The amount of K in KClO₃ = 1
So %K :