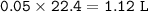Volume of O₂ = 1.12 L
Further explanation
The reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products
Reaction
4Na+O₂ ⇒ 2Na₂O
Ar Na = 23 g/mol
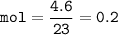
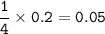
Assume at STP (0 C, 1 atm), 22.4 L for 1 mol , then volume of O₂ :