Answer:
The parent percentage is 93.75% and Daughter percentage is 6.25%
Step-by-step explanation:
we know , one complete life is 100%
Percentage of daughter in 1 half life = 50%
Percentage in 1/8 half lives=
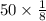
= 6.25%
Percentage of parent isotope = 100 - percentage of daughter's isotope
= 100 - 6.25%
= 93.75%
Hence , the parent percentage is 93.75% and Daughter percentage is 6.25%.