Answer:
At
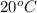
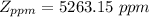
At
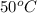
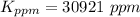
Step-by-step explanation:
From the question we are told that
The permissible exposure limit (PEL) for acetic anhydride is
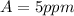
The exposure that is of immediate danger to life and health
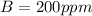
Gnerally the vapor pressure of acetic anhydride at
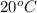 is
is
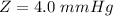
Now converting to ppm from the description in the question
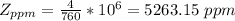
Gnerally the vapor pressure of acetic anhydride at
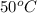 is
is
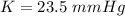
Now converting to ppm from the description in the question
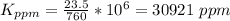
Note this vapor pressure are constant values