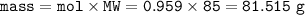mass of NaNO₃ : 81.515 g
Further explanation
The reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products
Reaction
HNO₃ + NaOH ⇒ NaNO₃ + H₂O
MW HNO₃ = 63 g/mol
MW NaNO₃ =85 g/mol
475 mL of 12% nitric acid (HNO₃)(density 1.06 g / mL)
Molarity HNO₃ :
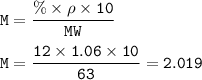
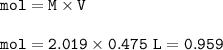
mol HNO₃ : mol NaNO₃ = 1 : 1 = 0.959