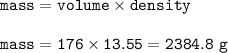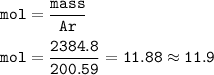Moles of Hg = 11.9
Further explanation
Density is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
The unit of density can be expressed in g/cm³ or kg/m³
Density formula:
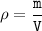
ρ = density
m = mass
V = volume
Density = 13.55 g/ml
volume = 176 ml