Answer:
The concentration of KOH solution is 17.009 moles/litre or 17.009M .
Step-by-step explanation:
Here , the given reaction is -
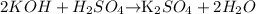
2moles of KOH requires 1mole for complete neutralisation.
Therefore ,
2 x number of moles of KOH = 1 x number of moles of
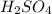
2 x (molarity of KOH x volume of KOH) = (Molarity of
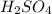 x volume of
x volume of
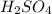 )
)
1 mole of
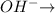 1mole of
1mole of

Molarity (KOH) = concentration (c)
volume (KOH) =15.05ML
Molarity (
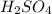 ) = 5.0 M
) = 5.0 M
Volume of (
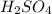 ) = 25.6ML
) = 25.6ML
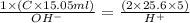
C =
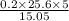
C= 17.009 moles/litre
Hence , the concentration of KOH solution is 17.009 M or moles/litre .