Answer:
The concentration of Mg²⁺ is 1.58x10⁻⁵ mol.l⁻¹
Step-by-step explanation:
Mg(OH)₂ ⇔ Mg²⁺ + 2OH⁻
The solution is said to be kept at pH 9.50
Since; pH + pOH = 14
Making pOH the subject,
∴ pOH = 14 - pH
= 14 - 9.50
= 4.50
since; pOH = -log[OH⁻]
∴ -log[OH⁻] = 4.50
[OH⁻] = Antilog (-4.5)
= Antilog (-4.5+5.5)
= 3.16 x 10⁻⁵ mol.l⁻¹
The chemical equation shows that [Mg²⁺] is half of OH ion concentration.
Therefore,
[Mg²⁺] =
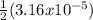
[Mg²⁺] = 1.58x10⁻⁵ mol.l⁻¹