Complete Question:
Jackie's uncle is undergoing radioactive iodine treatments for thyroid cancer. The radioactive iodine used by the doctor is an isotope of the element iodine. Jackie researches the term isotope. Which definition for the term does she learn?
Group of answer choices
A. atoms of the same element with a different number of neutrons
B. atoms of the same element with a different number of protons
C. atoms of the same element with a negative charge
D. atoms of the same element with a positive charge
Answer:
A. atoms of the same element with a different number of neutrons
Step-by-step explanation:
Isotopes can be defined as atoms of the same element with a different number of neutrons.
This ultimately implies that, the isotopes of an element have the same atomic number (number of protons) but different atomic mass (number of neutrons).
The isotope of an element is denoted by
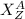
Where; X is the symbol of the chemical element.
A is the atomic mass or number of nucleons.
Z is the atomic number or number of protons.
Therefore, the number of neutrons = A - Z
In this scenario, radioactive iodine used by the doctor is an isotope of the element iodine.
Additionally, the isotopes of a chemical element have the same chemical properties because of their atomic number but different physical properties due to their atomic weight (mass number).