Answer:
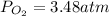
Step-by-step explanation:
Hello.
In this case, we need to use the Dalton's law equation and the ideal gas equation for this mixture containing oxygen, nitrogen and carbon dioxide:
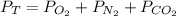

We know that the partial pressure of carbon dioxide is 0.250 atm and the moles of nitrogen are 0.210 mol, so we can compute its partial pressure via the ideal gas equation:

Thus the partial pressure of oxygen results:
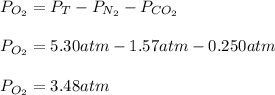
Best regards!