Answer:
![[A]_(eq)=0.854M](https://img.qammunity.org/2021/formulas/chemistry/college/915rq29f58i1nvf40mfhnmd97elaw237kx.png)
Step-by-step explanation:
Hello.
In this case, for the chemical reaction at equilibrium:
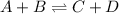
The equilibrium expression is:
![K=([C][D])/([A][B])=1.8](https://img.qammunity.org/2021/formulas/chemistry/college/wqztiy612pacgsmbq9wz74miiby4okwcbe.png)
Which in terms of the ICE methodology can be written as:
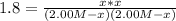
Solving for
 , we obtain:
, we obtain:
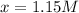
It means that the concentration of A once equilibrium is reached turns out:
![[A]_(eq)=2.00M-1.15M=0.854M](https://img.qammunity.org/2021/formulas/chemistry/college/ce217cc4knas38fn62x6pq3fkudjc7fej9.png)
Best regards.