Answer:
See Below.
Step-by-step explanation:
This is a conversion problem:
Using the molar mass of Gold (given in a periodic table) which is 196.97g/mol
you have
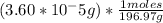
you always arrange the equation in a way to cancel whatever you don't want (grams) and leave what you do want (moles). Here grams cancel (top and bottom), so you're left with:
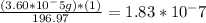 moles of Gold
moles of Gold