Answer:
V = 34.55 L
Step-by-step explanation:
Given that,
No of moles, n = 1.4
Temperature, T = 20°C = 20 + 273 = 293 K
Pressure, P = 0.974 atm
We need to find the volume of the gas. It can be calculated using Ideal gas equation which is :
PV=nRT
R is gas constant,
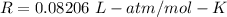
Finding for V,
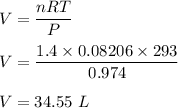
So, the volume of the gas is 34.55 L.