Answer:
Box A.
Step-by-step explanation:
Density can be defined as mass all over the volume of an object.
Simply stated, density is mass per unit volume of an object.
Mathematically, density is given by the equation;
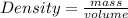
In this scenario, Box A and Box B have the same density. However, Box A had more volume. Therefore, the box that would have more mass would be Box A because it has more capacity or volume to hold more matter.
This ultimately implies that, the volume of a box is directly proportional to its mass (amount of matter it can hold); the higher the volume of the box, the higher its mass (more mass).
- Let's assume Box A has a volume of 25cm³ and a mass of 50kg.
It's density would be;
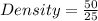
Density = 2kg/cm³
- Assuming Box B has a volume of 10cm³ and a mass of 20kg.
It's density would be;
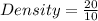
Density = 2kg/cm³
Therefore, it can be deduced that even though Box A and Box B have the same density; Box A had more volume and consequently, more mass.