Answer:
Silver.
Step-by-step explanation:
Hello!
In this case, we can consider the specific heat as the property that we can analyze in order to answer to this question. In such a way, as the specific heat is known as the energy required to modify the temperature of 1 g of the substance by 1 °C, since the masses of all the substances are the same, we can that their specific heats are respectively 0.240, 0.444 and 4.184 J/(g°C), from the equation:
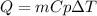
We can see that the higher the specific heat (Cp) the lower the change in temperature considering their inversely proportional relationship. However, as 100 J of energy is applied to all the substances, we can see that silver will exhibit the largest temperature change because a higher change is needed to fit with the provided energy.
Best regards.