Answer:
A) m
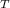 = 0.3025 * 0.0476 * 92.13 = 1.327 Ibm
= 0.3025 * 0.0476 * 92.13 = 1.327 Ibm
B) T= 63.32°F
Step-by-step explanation:
Given data:
1000 gallon tank currently contains 100.0 gallons of liquid toluene
and A gas saturated with toluene vapor at 85°F and 1 atm
A) Calculate quantity of toluene ( Ibm ) that will enter the atmosphere when the tank is filled
m
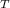 =
=
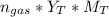
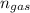 (total mole of gas) = 0.3025 Ib-mole ( calculated using :
(total mole of gas) = 0.3025 Ib-mole ( calculated using :
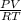 )
)
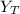 (mole fraction of toluene) = 0.0476 ( calculated using
(mole fraction of toluene) = 0.0476 ( calculated using
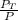 )
)
M
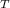 = 92.13 Ibm/Ib-mole
= 92.13 Ibm/Ib-mole
therefore: m
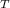 = 0.3025 * 0.0476 * 92.13 = 1.327 Ibm
= 0.3025 * 0.0476 * 92.13 = 1.327 Ibm
B) using Antoine equation to solve for T
Antoine equation :
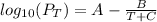
PT( partial pressure ) = 18.95 ( calculated using :
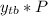 )
)
A = 6.95805
B = 1346.773
T = ?
C = 219.693
to calculate T make T the subject the subject of the equation
T + 219.693 = 1346.773 / 5.68044
∴ T = 17.40°C
convert T to Fahrenheit
T = 1.8 * 17.40 +32
= 63.32°F