Answer:
Step-by-step explanation:
A solution with a pH value of 7 is neutral i.e neither acidic nor alkaline. A solution with a pH less than 7 is said to be acidic, while one with a pH value more than 7 is alkaline. Acidity increases as the value decrease below 7, while alkalinity increases as the value increase about 7.
Given that H2A is a diprotic weak acid have pKa values of 5.0 ad 9.0, it will undergo dissociation in the solution as:
NOTE: These reactions are reversible reactions.
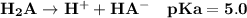
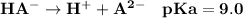
Thus;
The primary species is HA⁻
The secondary species is A²⁻