Answer:
V₂ = 87.24 L
Step-by-step explanation:
Charle's law states that at constant pressure, the volume of the gas is directly proportional to the temperature. Its mathematical form is given by :
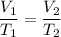
We have, V₁ = 75, T₁ = 245 K, T₂ = 285, V₂ = ?
Putting all the values, we get :
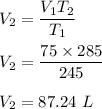
So, the new volume is 87.24 L.