Answer:
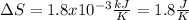
Step-by-step explanation:
Hello.
In this case, given the heat of fusion of THF to be 8.5 kJ/mol and freezing at -108.5 °C, for the required mass of 5.9 g, we can compute the entropy as:
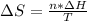
Whereas n accounts for the moles which are computed below:
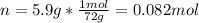
Thus, the entropy turns out:
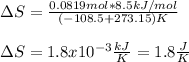
Best regards.