Answer:
1.
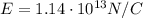
2.
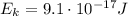
Step-by-step explanation:
1. The strength of the nucleus' electric field (E):
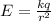
Where:
k: is the Coulomb constant = 9x10⁹ Nm²/C²
q: is the proton charge = 1.6x10⁻¹⁹ C
r: is the radius = 10⁻¹⁰ m
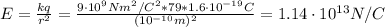
2. The kinetic energy (Ek) of an electron is the following:
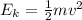
Where:
m is the electron's mass = 9.1x10⁻³¹ kg
v: is the speed of the electron
We can find the speed of the electron by equaling the centripetal force (Fc) and the electrostatic force (Fe):
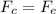
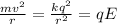
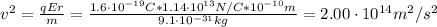
Now, we can find the kinetic energy:
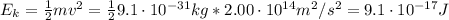
I hope it helps you!