Answer:
The concentration of chloride ion is
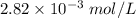
Step-by-step explanation:
We know that 1 ppm is equal to 1 mg/L.
So, the
 content 100 ppm suggests the presence of 100 mg of
content 100 ppm suggests the presence of 100 mg of
 in 1 L of solution.
in 1 L of solution.
The molar mass of
 is equal to the molar mass of Cl atom as the mass of the excess electron in
is equal to the molar mass of Cl atom as the mass of the excess electron in
 is negligible as compared to the mass of Cl atom.
is negligible as compared to the mass of Cl atom.
So, the molar mass of
 is 35.453 g/mol.
is 35.453 g/mol.
Number of moles = (Mass)/(Molar mass)
Hence, the number of moles (N) of
 present in 100 mg (0.100 g) of
present in 100 mg (0.100 g) of
 is calculated as shown below:
is calculated as shown below:
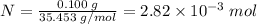
So, there is
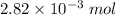 of
of
 present in 1 L of solution.
present in 1 L of solution.