Answer:
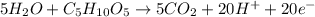
Step-by-step explanation:
Hello.
In this case, when ribose (C₅H₁₀O₅) yields carbon dioxide (CO₂) we write:
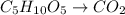
Which needs to be balanced by adding water and hydrogen ions:
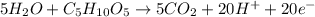
You can also see that there are 20 transferred electrons, since the carbon atoms in the ribose have 0 as their oxidation state and the carbon atoms in the carbon dioxide have +4 as the oxidation state, thus, each carbon transfers 4 electrons, a five carbon atoms transfer 20 electrons overall.
In such a way, since the carbon is increasing its oxidation state, such half reaction is an oxidation half reaction.
Best regards.