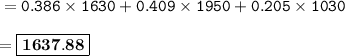The average atomic mass of the mystery element : 1637.88 amu
Further explanation
The elements in nature have several types of isotopes
Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
In determining the mass of an atom, as a standard is the mass of 1 carbon-12 atom whose mass is 12 amu
An atomic mass unit = amu is a relative atomic mass of 1/12 the mass of an atom of carbon-12.
The 'amu' unit has now been replaced with a unit of 'u' only
Mass atom X = mass isotope 1 . % + mass isotope 2.% ..
Isotope #1 - 85 particles, Isotope #2 - 90 particles, Isotope #3 - 45 particles, so total particles :
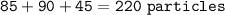
% particles :
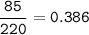
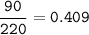
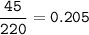
The average atomic mass of the mystery element