Given :
A reaction produces 2.5 moles of CO2 gas at STP.
To Find :
The volume of CO2 gas produced in the reaction.
Solution :
We know, in STP 1 mole of a gas contains 22.4 L of volume.
So, 2.5 moles of CO2 will produce :
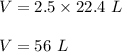
Therefore, volume of 2.5 moles CO2 is 56 L.
Hence, this is the required solution.