Answer:
The theoretical yield of CaS is 6.01 g.
Step-by-step explanation:
The balanced reaction is given as:
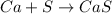
The molar mass of Ca and S is 40.08 and 32.065 g/mol respectively.
Number of moles =
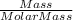
So, 7.19 g of Ca contains
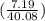 mol of Ca or 0.179 mol of Ca
mol of Ca or 0.179 mol of Ca
Also, 2.67 g of S contains
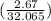 mol of S or 0.0833 mol of S
mol of S or 0.0833 mol of S
According to the balanced equation:
1 mol of Ca produces 1 mol of CaS
So, 0.179 mol of Ca produces 0.179 mol of CaS
According to the balanced equation:
1 mol of S produces 1 mol of CaS
So, 0.0833 mol of S produces 0.0833 mol of CaS
As the least number of mol of CaS (product) is produced from S , therefore, S is the limiting reactant.
So, thoretically, 0.0833 mol of CaS is produced.
The molar mass of CaS is 72.143 g/mol.
So, the mass of 0.0833 mol of CaS is
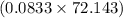 g or 6.01 g
g or 6.01 g
Hence, the theoretical yield of CaS is 6.01 g.