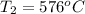Answer:
a
The pressure will increase
b
Step-by-step explanation:
From the ideal gas law we have that
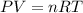
We see that the temperature varies directly with the pressure so if there is an increase in temperature that pressure will increase
The initial temperature is

The objective of this solution is to obtain the temperature of the gas where the pressure is tripled
Now from the above equation given that nR and V are constant we have that
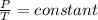
=>
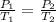
Let assume the initial pressure is
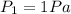
So tripling it will result to the pressure being
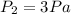
So
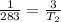
=>

=>

=>
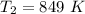
Converting back to
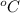
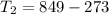
=>