Answer:
2.322 half-lives have passed to decay down from 150 miligrams to 30 miligrams.
Step-by-step explanation:
The half of Technetium-99 is approximately 211000 years. The decay of isotopes is represented by the following ordinary differential equation:
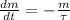 (Eq. 1)
(Eq. 1)
Where:
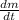 - First derivative of isotope mass in time, measured in miligrams per year.
- First derivative of isotope mass in time, measured in miligrams per year.
 - Mass of the isotope, measured in miligrams.
- Mass of the isotope, measured in miligrams.
 - Time constant, measured in years.
- Time constant, measured in years.
Now we proceed to obtain the solution of this differential equation:
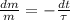
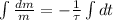
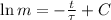
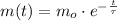 (Eq. 2)
(Eq. 2)
Where:
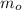 - Initial mass of the isotope, measured in miligrams.
- Initial mass of the isotope, measured in miligrams.
 - Time, measured in years.
- Time, measured in years.
The time passed for isotope is cleared within the equation described above:
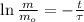
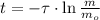
In addition, we can obtain the time constant as a function of half-life:
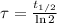 (Eq. 3)
(Eq. 3)
If we know that
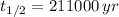 ,
,
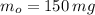 and
and
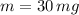 , then the time passed is:
, then the time passed is:
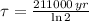
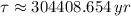
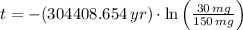
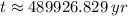
The amount of passed half-lives is that time divided by a half-life. That is:
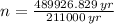
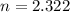
2.322 half-lives have passed to decay down from 150 miligrams to 30 miligrams.