Answer: The footprints were made
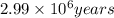 years long ago.
years long ago.
Step-by-step explanation:
Radioactive decay follows first order kinetics.
Half-life of isotope X= 500,000 years
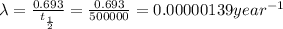
The equation for first order kinetics is :
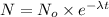
N = amount left after time t = 0.125 mg
 = initial amount = 8.00 mg
= initial amount = 8.00 mg
 = rate constant
= rate constant
t= time
Putting the values we get:
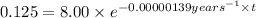

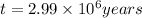
The footprints were made
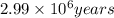 years long ago.
years long ago.