Answer:
The moles of gas is 0.009474 moles.
Step-by-step explanation:
Given that,
Volume = 526 mL
Pressure = 346 mmHg
Temperature = 35.0°C
We need to calculate the moles of gas
Using formula of ideal gas
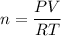
Where, P = pressure
V = volume
R = gas constant
T = temperature
Put the value into the formula
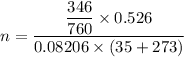
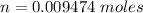
Hence, The moles of gas is 0.009474 moles.