Answer:
"0.053457 M" of sulfuric acid.
Step-by-step explanation:
The given values are:
 = 10 mL solution
= 10 mL solution
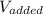 = 12.20 mL
= 12.20 mL
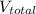 = 22.20 mL
= 22.20 mL
then,
M 0.103 M of NaOH,
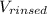 = experiment will not be affected
= experiment will not be affected
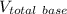 = 10.38 mL
= 10.38 mL
Now,
⇒ mol of NAOH = MV
=
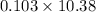
=
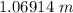
Whether Sulfuric acid, then
⇒
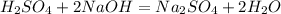
⇒
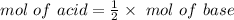
⇒
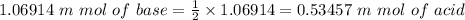
Before any dilution:
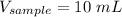
⇒
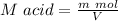
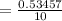
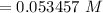 (Sulfuric acid)
(Sulfuric acid)