Answer:
The sample which would contain more oxygen atoms is a glass stirring rod.
Step-by-step explanation:
According to the periodic table ,
the glass stirring rod is Silicon dioxide and the cotton ball is cellulose
Here , the molar mass of the glass stirring rod
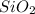 = 60.08 Grams/mole
= 60.08 Grams/mole
Molar mass of the cotton ball
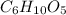 = 162.09 gram/mole .
= 162.09 gram/mole .
since ,total number of molecules present in oxygen is 32 ,
therefore ,
In glass stirring rod ,
number of oxygen atoms present in 5g =
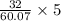
= 2.66 g of oxygen
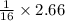
= 0.16625 moles
= 0.16625 x
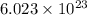
=
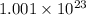 atoms
atoms
In cotton ball ,
number of oxygen atoms present in 5g =
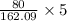
= 2.467 g of oxygen
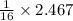
= 0.15418 moles
= 0.15418
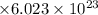
= 0.928
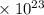
Hence , the glass stirring rod contains more number of oxygen atoms .