Answer:
hello your question lacks the required reaction pairs below are the missing pairs
Reaction system 1 :
A + B ⇒ D
![-r_(1A) = 10exp[-8000K/T]C_(A)C_(B)](https://img.qammunity.org/2021/formulas/chemistry/college/nl4vhx4q0skp8handc6t29yc3xvwinoahf.png)
A + B ⇒ U
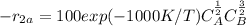
Reaction system 2
A + B ⇒ D
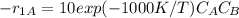
B + D ⇒ U
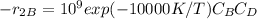
Answer : reaction 1 : description of the reactor system : The desired reaction which is the first reaction possess a higher activation energy and higher temperature is required to kickstart reaction 1
condition to maximize selectivity : To maximize selectivity the concentration of reaction 1 should be higher than that of reaction 2
reaction 2 :
description of reactor system : The desired reaction i.e. reaction 1 has a lower activation energy and lower temperatures is required to kickstart reaction 1
condition to maximize selectivity:
to increase selectivity the concentration of D should be minimal
Step-by-step explanation:
Reaction system 1 :
A + B ⇒ D
![-r_(1A) = 10exp[-8000K/T]C_(A)C_(B)](https://img.qammunity.org/2021/formulas/chemistry/college/nl4vhx4q0skp8handc6t29yc3xvwinoahf.png)
A + B ⇒ U
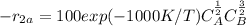
the selectivity of D is represented using the relationship below
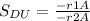
hence SDu = 1/10 *
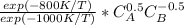
description of the reactor system : The desired reaction which is the first reaction possess a higher activation energy and higher temperature is required to kickstart reaction 1
condition to maximize selectivity : To maximize selectivity the concentration of reaction 1 should be higher than that of reaction 2
Reaction system 2
A + B ⇒ D
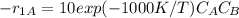
B + D ⇒ U
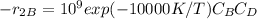
selectivity of D
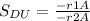
hence Sdu =
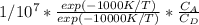
description of reactor system : The desired reaction i.e. reaction 1 has a lower activation energy and lower temperatures is required to kickstart reaction 1
condition to maximize selectivity:
to increase selectivity the concentration of D should be minimal