Answer:
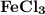 is the lewis acid
is the lewis acid
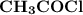 is the lewis base
is the lewis base
Step-by-step explanation:
The Lewis acid is a compound capable of accepting a doublet of electrons and which has an electronic gap on its valence layer.
The lewis base is a compound capable of donating a doublet of electrons.
A Lewis acid associates with a Lewis base through a covalent bond (sharing of two electrons between two atoms). The reaction between an acid and a Lewis base produces a compound called the Lewis adduct.
We can see the illustration of the diagrammatic expression in the image attached below.