Answer:
- The two gases will mix and react.: FALSE.
- The two gases will remain separate and will not mix: FALSE.
- The two gases will occupy a volume of 5.0 L and the final pressure in the -two bulbs will be 6.50 atm.: FALSE.
- The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 3.50 atm.: TRUE.
- The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 3.25 atm: FALSE.
Step-by-step explanation:
Hello.
In this case, given the options, since the total volume of the container includes the both of them, we find that:
- The two gases will mix and react: FALSE, since we do not know the identity of the gases which could be the same or two different inert gases.
- The two gases will remain separate and will not mix: FALSE, since as the valve is opened, the total gas will occupy the entire volume as the volume of a gas is the same to the container based on its constant molecules movements.
- The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 6.50 atm: FALSE, since in this case, by using the Boyle's law for the first compartment we obtain the pressure of the gas there:

Now, we reuse it for the gas at the 3.00-L bulb to find its final pressure:
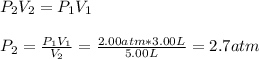
So the final pressure is:
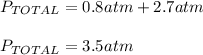
- The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 3.50 atm: TRUE considering the total pressure computed above.
- The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 3.25 atm: FALSE since the final pressure is 3.5 atm.
Regards.